Hangzhou administers first dose of groundbreaking ALS drug Tofersen
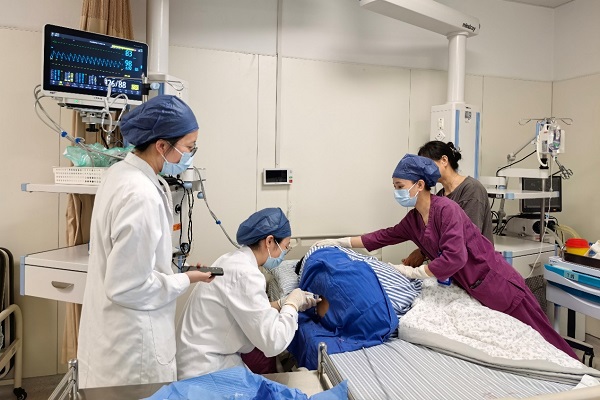
The first dose of the newly approved ALS drug Tofersen in Zhejiang is administered in Hangzhou. [Photo/tidenews.com.cn]
The first dose of the newly approved ALS (amyotrophic lateral sclerosis) drug Tofersen in Zhejiang province was administered at the ALS Center of Hangzhou First People's Hospital, affiliated with Westlake University School of Medicine, on June 10.
This marks the first use of the drug in the province and one of the first nationwide since its approval in October 2024.
Tofersen is the world's first treatment targeting the root cause of ALS in patients with SOD1 gene mutations. Unlike previous symptomatic treatments, Tofersen offers causal therapy — slowing disease progression by inhibiting the production of toxic SOD1 proteins that damage motor neurons.
ALS is a fatal neurodegenerative disease affecting nerve cells in the brain and spinal cord, leading to progressive muscle weakness and paralysis. China is home to one of the world's largest ALS patient populations, with over 40,000 cases, including more than 1,200 linked to SOD1 mutations.
Clinical trials suggest that Tofersen may significantly delay disease progression with manageable safety risks. Although currently applicable to only a small subset of ALS patients, its clinical rollout represents a major step toward precision medicine and offers new hope for targeted ALS treatment in China.
-
Visionary Pathway - Hangzhou Playbook
July 15, 2025



